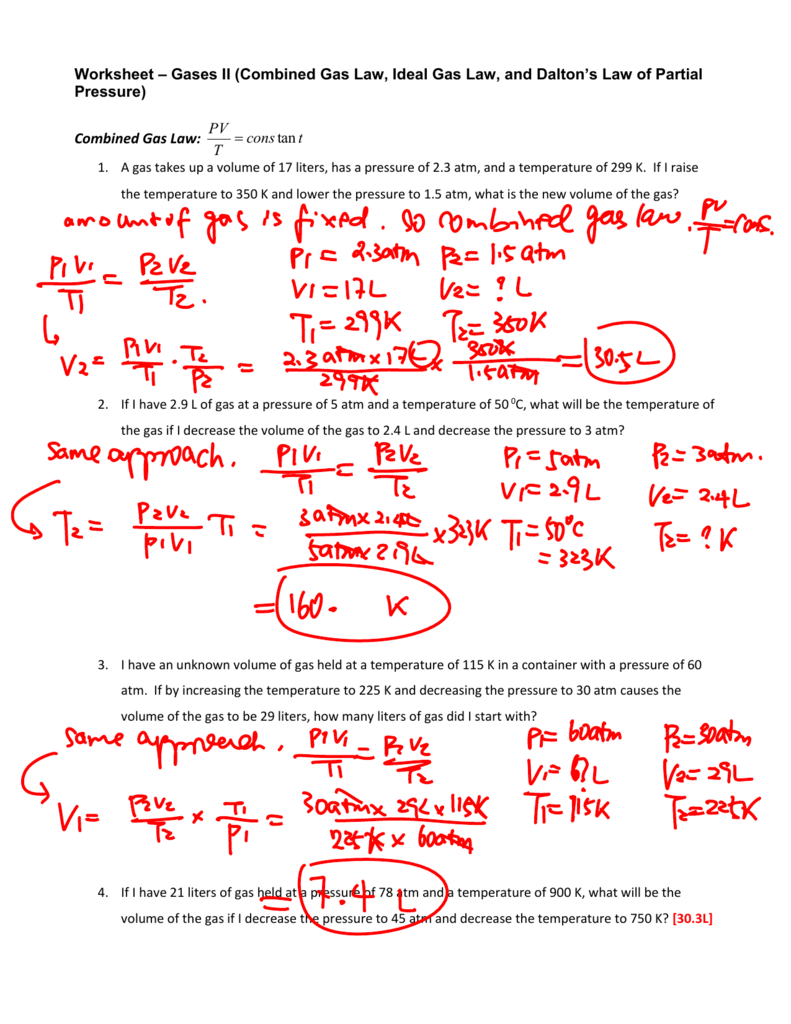
Combined Gas Law Questions And Answers Pdf . What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. use the combined gas law to solve the following problems: The combined gas law key solve the following problems. use the combined gas law to solve the following problems: mixed gas laws worksheet. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. What would the volume of the gas be at 227.0 °c and 600.0. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. What would the volume of the. If the temperature is raised to 29⁰c and the pressure does not. a gas has a temperature of 14⁰c, and a volume of 4.5 litres. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. As always, include enough work and show the units to.
from studylib.net
What would the volume of the gas be at 227.0 °c and 600.0. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. As always, include enough work and show the units to. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. a gas has a temperature of 14⁰c, and a volume of 4.5 litres. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. mixed gas laws worksheet. What would the volume of the.
Worksheet Gas Laws II Answers
Combined Gas Law Questions And Answers Pdf use the combined gas law to solve the following problems: The combined gas law key solve the following problems. mixed gas laws worksheet. a gas has a temperature of 14⁰c, and a volume of 4.5 litres. use the combined gas law to solve the following problems: As always, include enough work and show the units to. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. combined gas law practice problems 1. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. What would the volume of the gas be at 227.0 °c and 600.0. use the combined gas law to solve the following problems: 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. What would the volume of the.
From www.slideserve.com
PPT Gas Law Notes Chemistry Semester II PowerPoint Presentation, free Combined Gas Law Questions And Answers Pdf use the combined gas law to solve the following problems: As always, include enough work and show the units to. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. mixed gas. Combined Gas Law Questions And Answers Pdf.
From worksheetlibrarysteins.z19.web.core.windows.net
Combined Gas Law Problems Worksheet Combined Gas Law Questions And Answers Pdf The combined gas law key solve the following problems. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. use the combined gas law to solve the following problems: What would the volume of the.. Combined Gas Law Questions And Answers Pdf.
From printablelibmoits.z13.web.core.windows.net
Combined Gas Law Questions And Answers Combined Gas Law Questions And Answers Pdf What would the volume of the. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. The combined gas law key solve the following problems. a gas has a temperature of 14⁰c, and a volume of 4.5 litres. What volume will be occupied by 0.875 l of gas at 19 c after. Combined Gas Law Questions And Answers Pdf.
From www.semanarioangolense.net
Combined Gas Law Chem Worksheet 143 Answer Key » Semanario Worksheet Combined Gas Law Questions And Answers Pdf use the combined gas law to solve the following problems: A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. combined gas law practice problems 1. What would the volume of the gas be at 227.0 °c and 600.0. What volume will be occupied by 0.875 l of gas at 19 c after its. Combined Gas Law Questions And Answers Pdf.
From writersgroup749.web.fc2.com
Solving gas law problems Combined Gas Law Questions And Answers Pdf use the combined gas law to solve the following problems: 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. What would the volume of the. What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. Combined gas law 1 a gas. Combined Gas Law Questions And Answers Pdf.
From www.academia.edu
(DOC) ANSWER KEY for More Gas Law Practice Problems Ideal Gas Law Combined Gas Law Questions And Answers Pdf The combined gas law key solve the following problems. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. a gas has a temperature of 14⁰c, and a volume of 4.5 litres. mixed gas laws worksheet. use the combined gas law to solve the following problems: A gas. Combined Gas Law Questions And Answers Pdf.
From browsegrades.net
The Gas Laws Unit Test Review and Test Questions and Answers Scored 96 Combined Gas Law Questions And Answers Pdf What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. use the combined gas law to solve the following problems: 1) if i initially have a gas at a pressure of 12 atm, a volume. Combined Gas Law Questions And Answers Pdf.
From animalia-life.club
Combined Gas Law Worksheet Answers Combined Gas Law Questions And Answers Pdf What would the volume of the gas be at 227.0 °c and 600.0. use the combined gas law to solve the following problems: 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. a gas has a temperature of 14⁰c, and a volume of 4.5 litres. Combined gas law 1 a. Combined Gas Law Questions And Answers Pdf.
From worksheetdbenjamb.z21.web.core.windows.net
Combined Gas Law Questions And Answers Combined Gas Law Questions And Answers Pdf If the temperature is raised to 29⁰c and the pressure does not. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. use the combined gas law to solve the following problems: 1) how many. Combined Gas Law Questions And Answers Pdf.
From savekhaoyai.blogspot.com
40 chemistry combined gas law worksheet answers Worksheet Database Combined Gas Law Questions And Answers Pdf 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. The combined gas law key solve the following problems. use the combined gas law to solve the following problems: If the temperature is raised to 29⁰c and the pressure does not. 1) if i initially have a gas at. Combined Gas Law Questions And Answers Pdf.
From www.studocu.com
6 combined gas law worksheet Name Combined Gas Law Worksheet P 1 V 1 Combined Gas Law Questions And Answers Pdf combined gas law practice problems 1. What would the volume of the gas be at 227.0 °c and 600.0. As always, include enough work and show the units to. If the temperature is raised to 29⁰c and the pressure does not. What would the volume of the. 1) if i initially have a gas at a pressure of 12. Combined Gas Law Questions And Answers Pdf.
From studylib.net
Combined Gas Law Worksheet Combined Gas Law Questions And Answers Pdf What would the volume of the. combined gas law practice problems 1. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. As always, include enough work and show the units to. What volume will. Combined Gas Law Questions And Answers Pdf.
From animalia-life.club
Combined Gas Law Worksheet Answers Combined Gas Law Questions And Answers Pdf What would the volume of the gas be at 227.0 °c and 600.0. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. use the combined gas law to solve the following problems: What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. a. Combined Gas Law Questions And Answers Pdf.
From studylib.net
Gas Laws Worksheet Answer Key Combined Gas Law Questions And Answers Pdf a gas has a temperature of 14⁰c, and a volume of 4.5 litres. What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. use the combined gas law to solve the following problems: 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. Combined. Combined Gas Law Questions And Answers Pdf.
From www.studocu.com
Gas Laws Combined Gas Law Worksheet Combined Gas Law Worksheet Boyle Combined Gas Law Questions And Answers Pdf What would the volume of the. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. mixed gas laws worksheet. use the combined gas law to solve the following problems: combined gas law practice problems 1. a gas has a temperature of 14⁰c, and a volume of 4.5 litres.. Combined Gas Law Questions And Answers Pdf.
From lessoncampusplaguily.z13.web.core.windows.net
Grade 7 Reading Comprehension Worksheet Combined Gas Law Questions And Answers Pdf use the combined gas law to solve the following problems: The combined gas law key solve the following problems. What would the volume of the. mixed gas laws worksheet. As always, include enough work and show the units to. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. 1) how. Combined Gas Law Questions And Answers Pdf.
From app.formative.com
Ideal Gas Law Worksheet Alicia Barrett Library Formative Combined Gas Law Questions And Answers Pdf If the temperature is raised to 29⁰c and the pressure does not. The combined gas law key solve the following problems. use the combined gas law to solve the following problems: A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. What would the volume of the gas be at 227.0 °c and 600.0. As. Combined Gas Law Questions And Answers Pdf.
From studylibfreytag.z21.web.core.windows.net
Combined Gas Law Practice Worksheet Answers Combined Gas Law Questions And Answers Pdf What volume will be occupied by 0.875 l of gas at 19 c after its temperature is. What would the volume of the. combined gas law practice problems 1. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23. If the temperature is raised to 29⁰c and the pressure does not. . Combined Gas Law Questions And Answers Pdf.